FDA recalls some birth control pills with wrong instructions
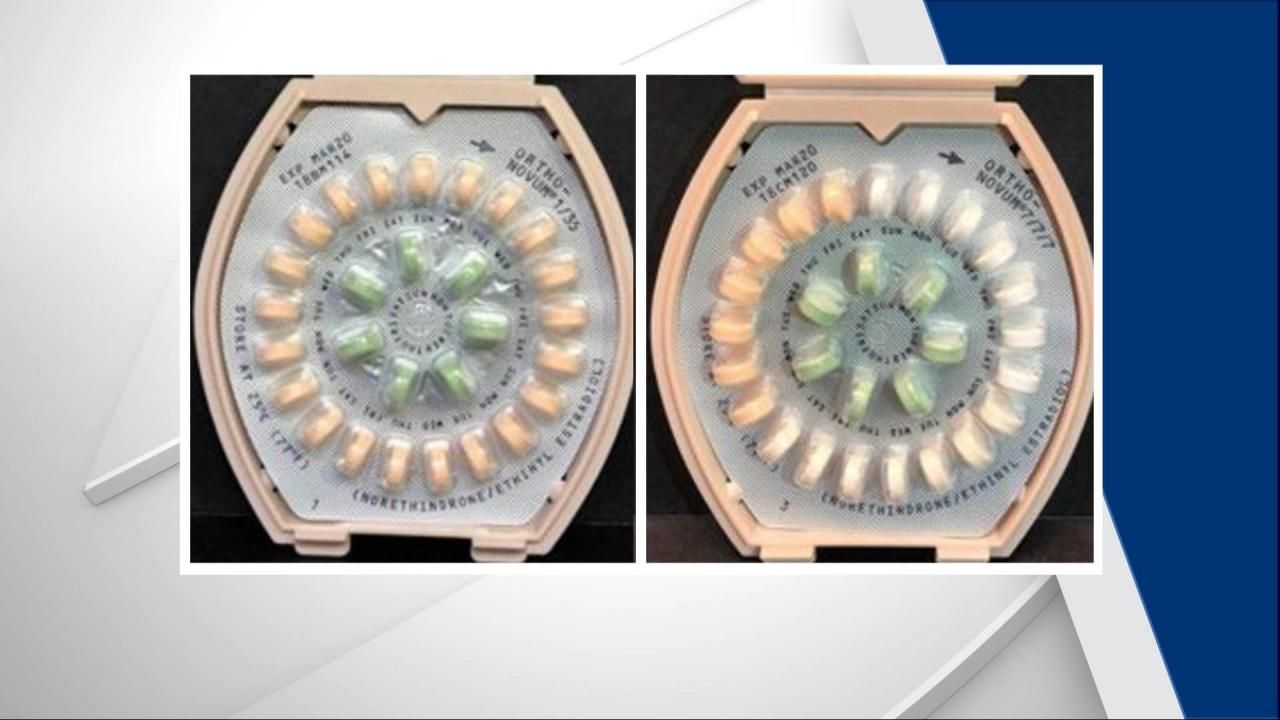
Ortho-Novum has voluntarily recalled three lots of birth control pills due to incorrect instructions.
According to the FDA, the recall includes Ortho-Novum 1/35 norethindrone/ethinyl estradiol tablets and two lots of Ortho-Novum 7/7/7 norethindrone/ethinyl estradiol tablets because the instructions are incorrect. All three have expiration dates of March 2020.
Consumers could take the incorrect “inactive” pill instead of an active pill if they follow the directions included in these lots, which could lead to breakthrough bleeding or an unintended pregnancy, according to the FDA.
Products were distributed in the U.S. to wholesalers, distributors and pharmacies, and they have been notified by recall letter to return affected products.
Users should contact their healthcare professional with any concerns.
The company advises consumers to not stop taking the pills, and to find the correct instructions online. Consumers can also call 1-800-526-7736 Monday through Friday from 9 a.m. to 8 p.m. ET.









